Virongy focuses on developing cutting-edge technologies in the field of virology and viral vectors. Our goal is to develop new technologies that can be used for scientific discovery, clinical diagnostics, and disease treatment. Currently, Virongy has developed the following key technologies:
InfectinTM
InfectinTM is a proprietary technology developed at Virongy and based on the scientific theory that cortical actin is a natural barrier for viral entry and intracellular migration towards the cytosol and the nucleus (Yoder et al., 2008). Cortical actin is a dense meshwork of actin filaments (F-actin) underneath the plasma membrane. It provides mechanical support to cells and is a major driving force for cell motility. As a fundamental component of the host cells, cortical actin represents a structural impediment for viral intracellular migration towards sites where viruses replicate and assemble. Viruses devise strategies to exploit various cellular regulatory mechanisms of actin dynamics in order to overcome the cortical actin barrier. For example, in human immunodeficiency virus (HIV) infection of CD4 T cells, the virus utilizes chemokine co-receptor (CXCR4 or CCR5) signaling to promote cortical actin treadmilling in order to facilitate viral migration towards the nucleus (Yoder et al., 2008). Many viruses use endocytosis to penetrate the cortical actin meshwork. The endosomal entry of viruses also requires active actin polymerization to drive endosome scission from the plasma membrane.
InfectinTM is designed to increase actin dynamics to facilitate viral penetration of the cortical actin barrier, thereby greatly facilitating productive viral infection. InfectinTM can enhance the numbers of productively infected cells by 5- to 20-fold. It can be used to drastically increase the viral infection rate to facilitate biochemical characterization of viral infection. InfectinTM can be used as a routine viral infection-enhancing reagent to increase productive viral vector transduction rates, particularly for common vectors such as lenti- and retroviral vectors.
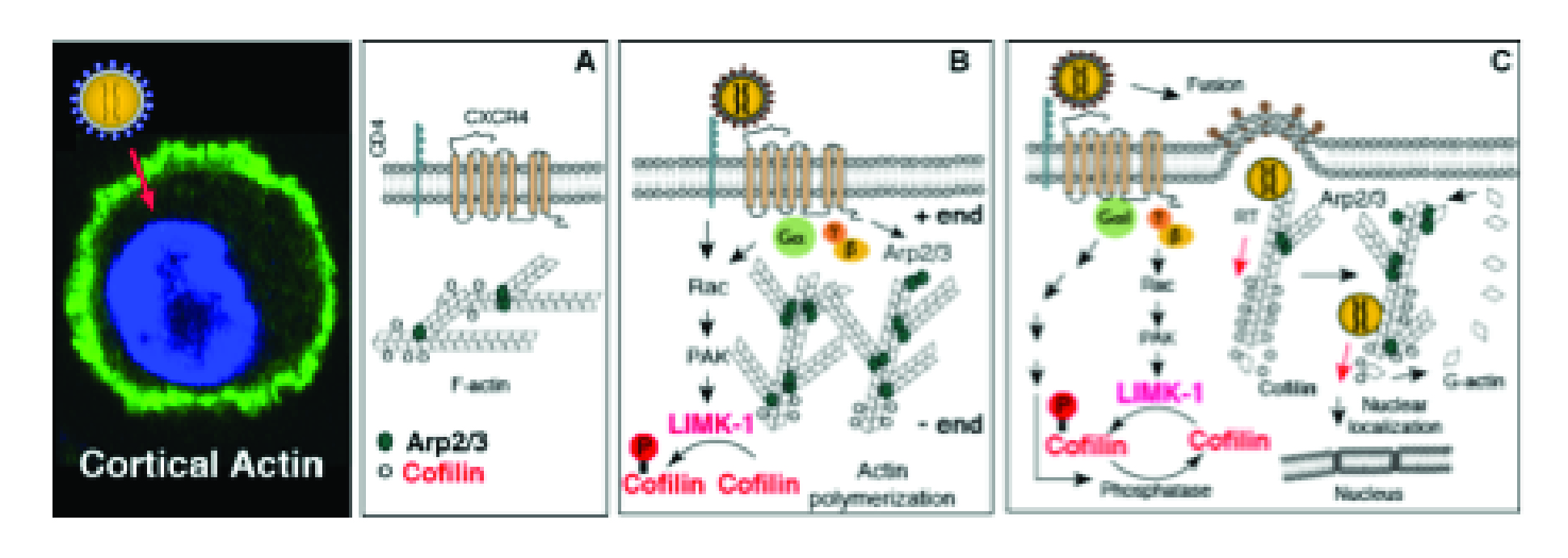 Model of HIV exploitation of actin dynamics to overcome the cortical actin barrier. Cortical actin (green staining, F-actin) in blood CD4 T cells remains relatively static in the absence of chemotactic signaling. Binding of viral envelope protein gp120 to CXCR4/CCR5 triggers a transient course of actin polymerization and depolymerization, through the activation of the cofilin/Arp2/3 pathways, to facilitate viral entry and post-entry nuclear migration.
Model of HIV exploitation of actin dynamics to overcome the cortical actin barrier. Cortical actin (green staining, F-actin) in blood CD4 T cells remains relatively static in the absence of chemotactic signaling. Binding of viral envelope protein gp120 to CXCR4/CCR5 triggers a transient course of actin polymerization and depolymerization, through the activation of the cofilin/Arp2/3 pathways, to facilitate viral entry and post-entry nuclear migration.
The LIM kinase inhibitor R10015
LIM domain kinases (LIMKs) are serine/threonine and tyrosine kinases which phosphorylate and inactivate the ADF/cofilin family of proteins through serine 3 phosphorylation (Yang et al., 1998). LIMKs regulate actin dynamics by phosphorylating cofilin, thereby inhibiting cofilin-mediated depolymerization of the actin filaments. LIMKs are involved in many cellular processes associated with actin dynamics and actin cytoskeletal rearrangement, such as cell migration, chemotaxis, cell development, neuronal differentiation, cell-cell interaction, tissue repair, wound healing, cancer metastasis, and viral infection (Foletta et al., 2004; Manetti, 2012; Yoshioka et al., 2003). LIMK is highly expressed in many metastatic cancer cells, and has been identified as a possible therapeutic target (Endo et al., 2007; Nishimura et al., 2006). In addition, LIMK has been shown to be involved in the infection and pathogenesis of multiple viruses such as HIV, influenza A, pseudorabies virus, EBOV, RVFV, VEEV, and HSV-1 (Liu et al., 2014; Vorster et al., 2011). LIMK inhibitors have also been shown to possess broad-spectrum antiviral activities.
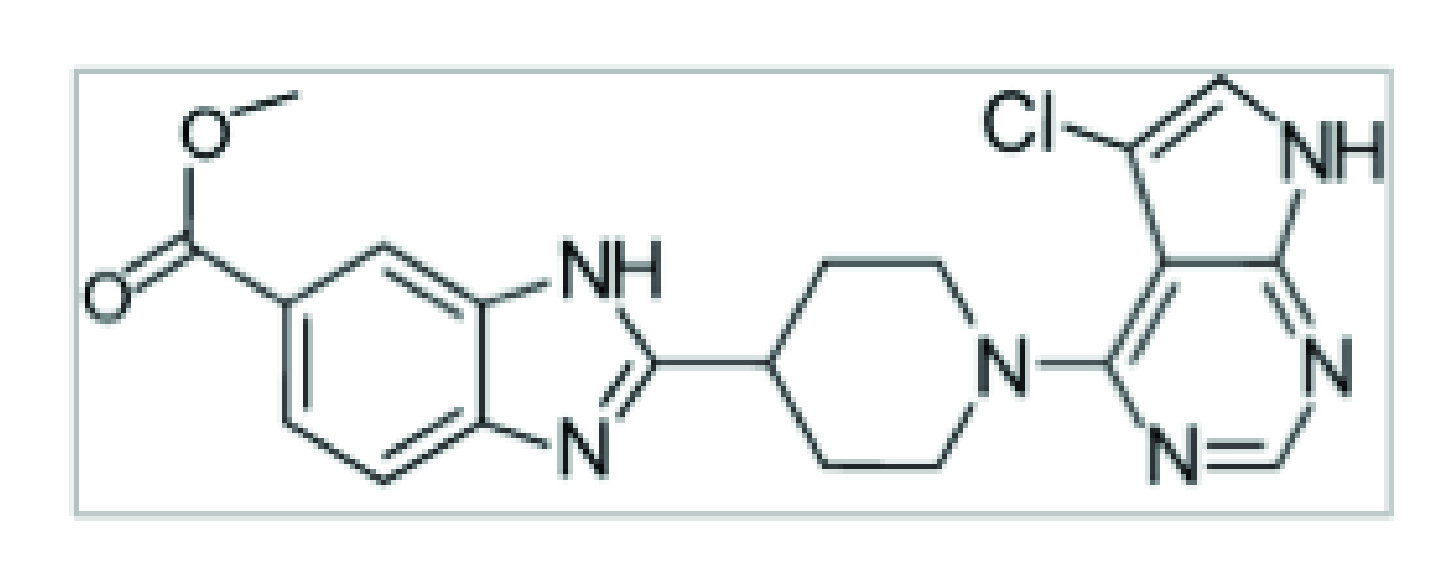
The LIMK inhibitor R10015, licensed and characterized by Virongy, is the best biochemically and biologically characterized LIMK inhibitor (Yi et al., 2017). R10015 directly inhibits the kinase activity of LIMK by binding to the ATP-binding pocket of LIMK. The in vitro IC50 value for inhibiting recombinant LIMK1 protein is approximately 38 nM. R10015 blocks the phosphorylation of cofilin at serine 3 in human T cells. R10015 also inhibits T cell chemotaxis and chemokine-induced actin polymerization.
R10015
HIV Rev-dependent reporter cells
The HIV Rev-dependent reporter cells licensed and commercialized by Virongy represent a major advancement in the development of HIV indicator cells (Wu et al., 2007). This new reporter system differs dramatically from the common LTR-based reporter cells, which rely solely on the HIV promoter, the long terminal repeat (LTR), to drive reporter expression. While responsive to an early HIV protein, Tat, the LTR is also responsive to cell culture conditions and stimulation by a variety of known and unknown factors, including cytokines, mitogens, HDAC inhibitors, lipopolysaccharide, certain anti-tumor drugs, and free viral proteins (Siekevitz et al., 1987; Sweet et al., 1995). Such non-HIV-dependent reporter expression frequently diminishes reporter specificity and sensitivity. In contrast to the LTR-based reporter cells, our Rev-dependent reporter cells use both LTR and the Rev/RRE interaction to regulate reporter gene expression. This strict requirement for Rev, a viral protein present only in infected cells, drastically improves the reporter specificity and sensitivity. This unparalleled sensitivity and specificity means that our Rev-dependent reporter cells are suitable for a broad range of applications, including screening broadly neutralizing antibodies and anti-HIV drugs, and studying HIV cell-cell transmission and host restriction and dependency factors. Derived from CD4 T-cells, our reporter cells express native levels of HIV receptors, and are natural HIV targets with broad susceptibility to X4, R5, primary HIV isolates, and certain SIV strains. With GFP, Luc, or GFP/Luc detection options, our Rev-dependent cells provide a versatile and flexible platform for HIV research.
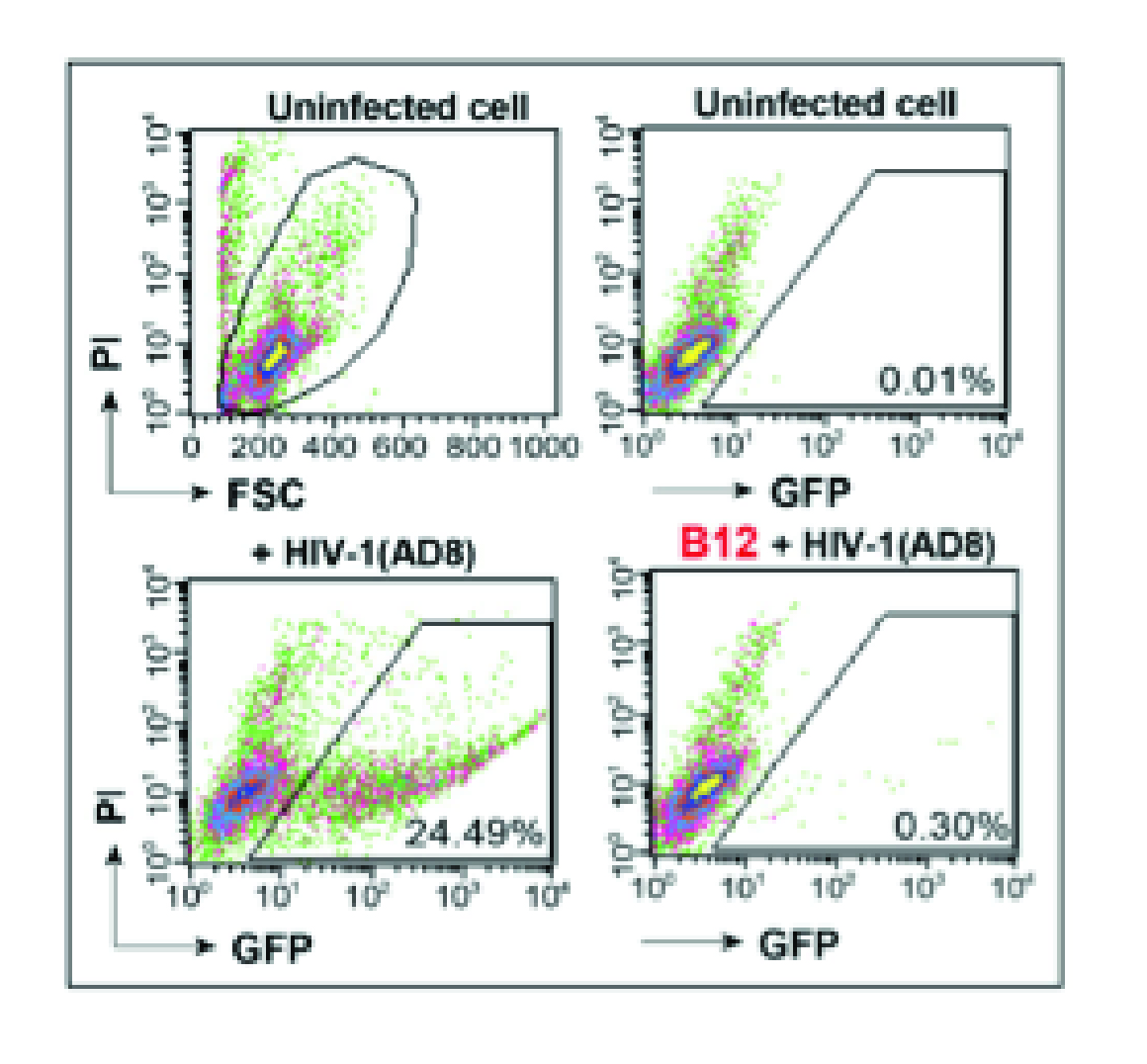
Quantification of anti-HIV neutralizing antibody B12 with Rev-A3R5-GFP reporter cells
References
